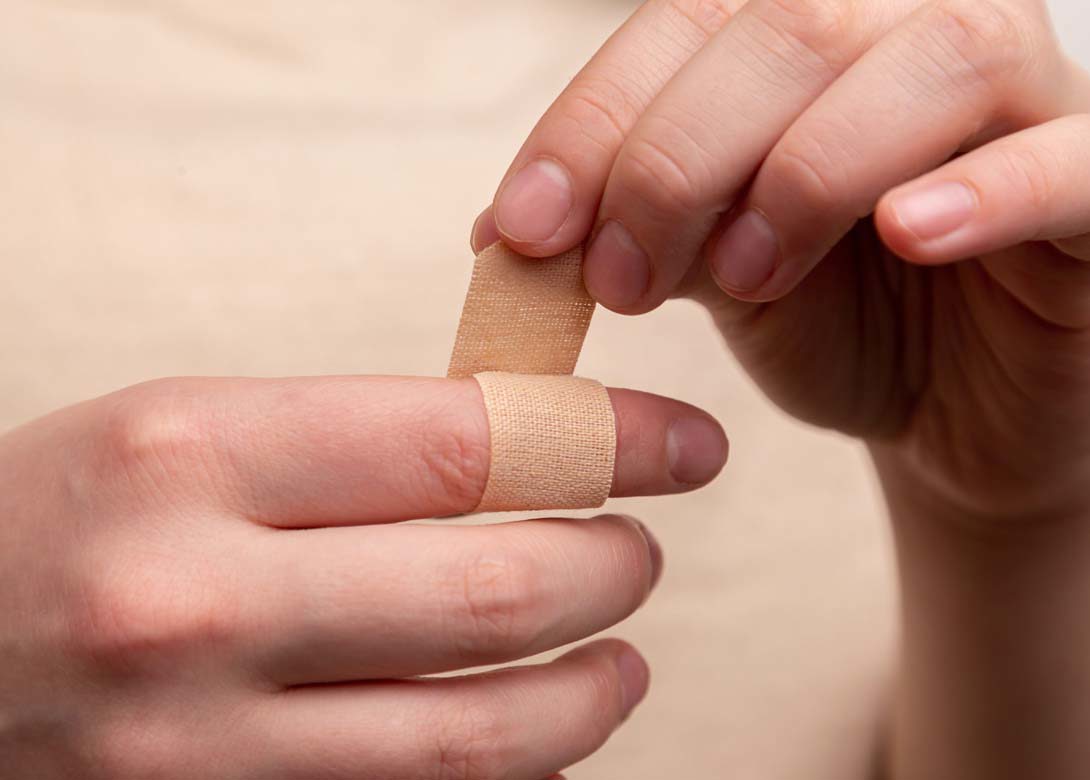
Over the last few years and during the global Covid-19 pandemic, the demand for wearables applications in the medical industry has significantly increased. As sensitive skin demands for sensitive and flexible products, Lohmann has designed and released a new portfolio of bonding solutions offering high breathability, water resistance, as well as a residue-free and painless removability.
With highly demanding requirements to the long-lasting adhesion to skin, permanent changes and fluctuating external influences, low surface energy, rough textures or sweat, the Lohmann wearables adhesive solutions are engineered and tailored to medical industry's applications and design requirements.
No matter if applied to mobile cardiac telemetry (MCT), continuous glucose or temperature measurement (CGM and CTM), insulin patch pumps or large volume injectors, for short or long-term use, single or double-sided, each bonding solution is biocompatible and allows for ever thinner and more flexible wearables. This gives patients a higher level of comfort and mobility.
The Lohmann product portfolio supports connectivity features of wearables via electrical conductivity, i.e for quantifying symptoms via (remote) monitoring devices and thus predicting and preventing future acute episodes. As patients are becoming stakeholders in their own healthcare journey due to remote monitoring and possible recovery at home, costs for the healthcare system, which is affected by nursing shortage and shrinking budgets, are significantly reduced, too. An increasingly important aspect in an ageing population and with chronic diseases on the rise.
Meeting the needs of the medical and healthcare industry, Lohmann provides in-house state of the art capabilities, which include adhesive formulation, coating, casting and extrusion as well as clean room conversion, laboratories and testing as well as die cutting. The Neuwied-based adhesive technology specialist is certified in accordance with EN ISO 13485.

Biog
Having spent a decade in the fastener industry experiencing every facet – from steel mills, fastener manufacturers, wholesalers, distributors, as well as machinery builders and plating + coating companies, Claire has developed an in-depth knowledge of all things fasteners.
Alongside visiting numerous companies, exhibitions and conferences around the world, Claire has also interviewed high profile figures – focusing on key topics impacting the sector and making sure readers stay up to date with the latest developments within the industry.






